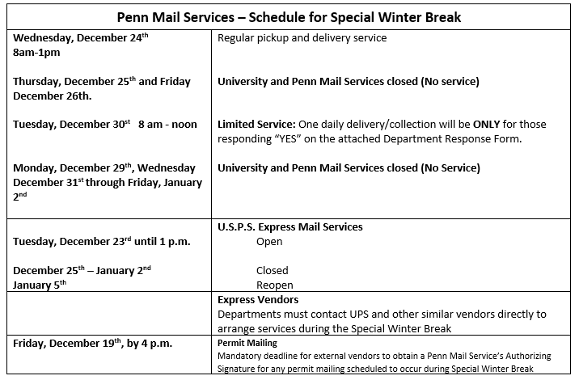
Medical Miracles at Penn Medicine: Penn Medicine Surgeons Led Teams That Performed Historic Double Hand Transplant

When 29-year-old Luka Krizanac reflects on the long chapter of his life without hands, he emphasizes that it wasn’t an entirely sad one.
Those 16 years in Zurich, Switzerland, were filled with moments of beauty and personal fulfillment. Mr. Krizanac (pronounced Kree-JAH-nahtz) took up drawing and painting, graduated from college, received a master’s degree in political science and business administration, got a job in banking, traveled with his family, and spent glorious summers by the sea in Croatia. Through the years, he had immeasurable support from his parents and brother. Yet, the loss of his hands at age 12 was a trauma and tragedy that robbed him of independence during those teen and early adult years.
“People usually struggle to understand how much they do with their hands,” he said. “And I don’t mean just practical stuff, but basically surviving as humans, even in today’s modern world. As much as you try to build the comfort and confidence without hands...you always have someone assisting you. As much as you love them, as much as you care about them, you never have the chance to do it on your own, which ties into the fact that you’re also not able to fully develop as a person.”
In the fall of 2024, Mr. Krizanac received the gift of new hands at Penn Medicine. Long before he regained sensation or functionality in the new limbs, he said, he began to feel like “a whole human being.”
Penn hand transplant program director and orthopaedic and plastic surgeon L. Scott Levin, with plastic surgeon Benjamin Chang, the program’s co-director, worked with four surgical teams for more than 10 hours at the Hospital of the University of Pennsylvania to connect donor forearms and hands to Mr. Krizanac’s upper arms.
Mr. Krizanac was Penn Medicine’s first bilateral hand and forearm transplant recipient since before the COVID pandemic and the first in the U.S. since 2021. His was the fifth such transplant performed by the vascularized composite allotransplantation team led by Drs. Levin and Chang.
Penn’s hand transplant program is one of only a few in the world. Dr. Levin established the program with liver transplant surgeon Abraham Shaked in 2009, as part of the Penn Transplant Institute, with support from liver, kidney, and pancreas transplant surgeon Matthew H. Levine and liver transplant surgeon Kim Olthoff.
For Mr. Krizanac, the journey to receive hands spanned more than 16 years, a global pandemic, a fateful connection between a surgeon and his mentor, and surgeries on two continents.
A Devastating Loss
Mr. Krizanac was on the cusp of adolescence in 2008 when an improperly treated infection led to severe sepsis and multi-organ failure that almost killed him. Surgeons at another hospital amputated parts of both his arms and legs to save his life.
He learned early on to use prosthetic legs, and they served him well in daily life; he never thought about not having legs. But hands were a different story. Prosthetics didn’t come close to replicating the intricate movements and flexibility of the real thing.
“A lower leg is less complex than the human hand,” Dr. Levin said, noting that the lower extremity is primarily used for standing and walking. On the other hand, “you do 1,001 activities on a daily basis with your hands. Upper extremity prosthetics usually cannot provide that degree of function.”
Mr. Krizanac learned to eat, type, draw, and do many things for himself at home without prosthetic hands, and he reserved the prosthetics for when he left the house.
But Mr. Krizanac couldn’t hold a pen and take notes with a silicone hand or slip on a jacket without the prosthetics getting stuck in his sleeves. It wasn’t possible to run a silicone hand through his hair and not tear out the hair. Nor could he go to a cafe on his own and buy himself a coffee, with all the steps the trip entailed: Put on his jacket. Open his wallet. Hold the cup. Take off the lid to add a bit of cold milk. He required assistance for so many of the tasks many people rely on their hands to do without thinking.
“For everyone else, this is a daily thing that they don’t even think about—they forget about it the instant that they do it,” he said, thinking about the coffee shop. He mentioned the example, not because he cared that much about going out for coffee, but to illustrate the independence it represented.
A Fateful Connection
Not long after his illness, his mother read about a patient who received a hand transplant in Austria. He and his parents would sit in countless doctors’ waiting rooms, pursuing a hand transplant in their country and making no progress.
Meanwhile, in 2016, Dr. Levin traveled to Switzerland’s Balgrist University Hospital to give a lecture about the program he and Dr. Chang had built at Penn; at that point, they had performed two bilateral hand transplants, including the world’s first on a child, a collaboration between Penn Medicine and Children’s Hospital of Philadelphia. The lecture Dr. Levin gave that day was named for Reinhold Ganz—a pioneering hip surgeon and Dr. Levin’s mentor during a specialized orthopaedics fellowship in Switzerland in 1988. Dr. Ganz was in the audience as Dr. Levin spoke.
Two years later, when Mr. Krizanac’s family was facing roadblocks, Dr. Ganz called his former fellow. Mr. Krizanac’s father, a nurse, was the operating room manager at the medical center where the renowned surgeon worked.
Getting a referral from his former mentor “was a tremendous honor, but also a tremendous responsibility,” Dr. Levin said. “He’s a remarkable guy and has done so much for thousands of patients.” Being given the chance by Dr. Ganz to perform the first vascularized composite allotransplant—the term for the transplantation of multiple tissue types as a single functional unit—of a Swiss patient was particularly meaningful. Mr. Krizanac was 22 years old and had been living without hands for a decade.
“As you can imagine, especially as a kid, you are disappointed in doctors,” Mr. Krizanac said. “You’re disappointed in health care. You feel betrayed by that. But then, to encounter someone who is so dedicated to you, so dedicated to helping you and making your life better, this just changes your whole perspective on the medical field and what it means to be a patient.”
The Penn hand transplant team began the labor-intensive process of rehearsing for his transplant in Philadelphia. Unlike other surgeries, each hand transplant is customized to the patient. Attending surgeons, residents, fellows, and nurses rehearsed Mr. Krizanac’s surgery more than 12 times. They donated their time after-hours to rehearsals in the cadaver lab, continuing through the COVID pandemic so they would be ready to go when a match was found.
A Complication and a Committed Team
Mr. Krizanac’s belief in Dr. Levin and his team was cemented when he developed painful wounds on his knees and required reconstructive surgery before the hand transplants could go forward. Transplant recipients must take immunosuppressant drugs continuously to stop their bodies from rejecting the foreign limbs, and the immunosuppression would impair the wound-healing process on his legs.
In April 2021, Dr. Levin and Penn Medicine plastic surgeon Stephen J. Kovach, III, co-director of the Penn Orthoplastic Limb Salvage Center, flew to Switzerland to reconstruct Mr. Krizanac’s lower limbs. They worked in two teams at the University Hospital of Bern, with Esther Vögelin and Radu Olariu, the hospital’s co-director of hand and plastic surgery and co-chief of plastic surgery. The surgery took seven hours.
“Luka saw in our team that we were committed to him for the long haul,” Dr. Chang said.
It wasn’t just that Dr. Levin was willing to cross an ocean for Mr. Krizanac. The surgeon treated him as more than a patient, he said.
“When I came out of the surgery for the legs, people would talk to me standing at the end of the bed,” Mr. Krizanac said. “He would kneel to be the same height as me. He’s a person who is very senior, who has a huge, huge amount of knowledge and talent; but when he speaks to me, he’s like, ‘What is worrying you? What can I explain? What has someone said that is unclear?’”
After a year of recovery, Mr. Krizanac was ready for hands.

The Wait
Through the years, Mr. Krizanac remained hopeful that there was a light at the end of his tunnel—even if he didn’t know how long it would take to get there. But desperation set in at times, knowing there was a solution out there—but not knowing when, or if, it would come to him.
Then, last fall, Dr. Levin and Mr. Krizanac agreed that he should come to Philadelphia to wait for a donor. Mr. Krizanac was the only one on the national waiting list for a bilateral hand transplant, Dr. Levin said, and he was “the perfect candidate.”
But the days passed by. Finding the right set of arms and hands is complicated—in addition to matching blood and tissue types, they must also match for gender, skin color and tone, and size.
Mr. Krizanac and his family felt the ongoing support of not only Dr. Levin, but the whole transplant team, along with Dr. Levin’s wife, Helga, who is German. She baked German bread for Mr. Krizanac’s family each week and made them feel at home. Each day, the surgeon remembers asking her, “When is this going to happen? We must find a donor for Luka.”
Finally, after eight weeks, the call came. There was a match.
At 1:34 a.m., Mr. Krizanac went into surgery. Like all hand transplants, it was a complicated operation, involving four surgical teams—two on the donor limbs, and two on the recipient’s—working through the night to carefully connect the bones, nerves, arteries, muscles, and skin.
Feeling the Weight of New Hands
Transplant nurse Charlotte “Carly” Baker, remembers a day, a couple of weeks after the surgery, when the physical therapy team put Mr. Krizanac’s new arms into slings and had him walk.
“He didn’t let anyone rush him in the moment; it seemed like he wanted to soak it all in, as he felt where his new arms and hands were,” she said. “That moment is something that will stick with me forever, because I was very amazed at his ability to be really mindful.”
Just three weeks after the surgery, Mr. Krizanac could push up his glasses and scratch his cheek using his own fingertip. And less than a week after that, Dr. Levin received a video on his phone from Mr. Krizanac. It showed his patient using his phone with his new hand.
“That was really incredible … to get that video,” Dr. Levin said. “All the nights, all the rehearsals, all the anxiety, all the preparation—of course, it’s worth it.”
Dr. Levin emphasized that the outcome would not have been possible without the close collaboration with his Swiss colleagues—from Dr. Ganz’s referral of Mr. Krizanac to Dr. Vögelin and her team’s partnership, first for Mr. Krizanac’s leg surgery, and continuing through his ongoing physical therapy and recovery.
In fact, Dr. Vögelin visited Philadelphia for two weeks while Mr. Krizanac was waiting, hoping the right donor would be found during that time. Having performed research in the field of VCA, the Swiss surgeon hoped to attend the complex surgery of bilateral forearm transplantation, she said. While she couldn’t be at the surgery, she was grateful for the time spent in Philadelphia with Mr. Krizanac, his family, and the whole Penn team.
“Such international collaborations are very stimulating because of the exchange of knowledge in another environment,” Dr. Vögelin said. Being in Philadelphia “also established a relationship of trust for an optimal aftercare in Switzerland.”
Five months later, Mr. Krizanac leaned his head on his hand as he spoke—something he could never do with prosthetics. He could feel cold water on his hands. And he could almost put his jacket on without assistance. He continues to undergo several hours of physical and occupational therapy each week to practice using his new hands.
“He has waited so long for his ‘new hands’—we experience his devotion and motivation towards his rehabilitation with every visit,” Dr. Vögelin said, adding that Mr. Krizanac “has had an amazing recovery so far.”
Over the next few years, his nerves and muscles will gradually regrow into the transplanted hands to give him more feeling and function. Mr. Krizanac knows he will achieve the independence he has longed for. For now, he said, resting his hand on his upper arm, “feeling full sensation, and just feeling fingers that are not made out of silicone, is still mind-blowing.”
Adapted from a Penn Medicine article by Daphne Sashin, June 13, 2025.
Medical Miracles at Penn Medicine: Breakthrough with Customized CRISPR Treatment for Patient with CPS1

In a historic medical breakthrough, a child diagnosed with a rare genetic disorder has been successfully treated with a customized CRISPR gene editing therapy by a team at Children’s Hospital of Philadelphia (CHOP) and Penn Medicine. The infant, KJ, was born with a rare metabolic disease known as severe carbamoyl phosphate synthetase 1 (CPS1) deficiency. After spending the first several months of his life in the hospital and on a very restrictive diet, KJ received the first dose of his bespoke therapy in February 2025, when he was between six and seven months of age. The treatment was administered safely, and he is now growing well and thriving.
The case is detailed in a study published by the New England Journal of Medicine and was presented at the American Society of Gene & Cell Therapy Annual Meeting in New Orleans. This landmark finding could provide a pathway for gene editing technology to be successfully adapted to treat individuals with rare diseases for whom no medical treatments are available.
“Years and years of progress in gene editing and collaboration between researchers and clinicians made this moment possible, and while KJ is just one patient, we hope he is the first of many to benefit from a methodology that can be scaled to fit an individual patient’s needs,” said Rebecca Ahrens-Nicklas, director of the Gene Therapy for Inherited Metabolic Disorders Frontier Program (GTIMD) at Children’s Hospital of Philadelphia and an assistant professor of pediatrics in the Perelman School of Medicine.
CRISPR (clustered regularly-interspaced short palindromic repeats)-based gene editing can precisely correct disease-causing variants in the human genome. Gene editing tools are incredibly complex and nuanced, and up to this point, researchers have built them to target more common diseases that affect tens or hundreds of thousands of patients, such as the two diseases for which there currently are U.S. Food and Drug Administration-approved therapies, sickle cell disease and beta thalassemia. However, relatively few diseases benefit from a “one-size-fits-all” gene editing approach, since so many disease-causing variants exist. Even as the field advances, many patients with rare genetic diseases—collectively impacting millions of patients worldwide—have been left behind.
A Collaborative Effort
Drs. Ahrens-Nicklas and Kiran Musunuru, the Barry J. Gertz Professor for Translational Research in Penn’s Perelman School of Medicine, who are co-corresponding authors of the published report, began collaborating to study the feasibility of creating customized gene editing therapies for individual patients in 2023, building upon many years of research into rare metabolic disorders, as well as the feasibility of gene editing to treat patients. Both are members of the NIH-funded Somatic Cell Genome Editing Consortium, which supports collaborative genome editing research.
Drs. Ahrens-Nicklas and Musunuru decided to focus on urea cycle disorders. During the normal breakdown of proteins in the body, ammonia is naturally produced. Typically, our bodies know to convert the ammonia to urea and then excrete that urea through urination. However, a child with a urea cycle disorder lacks an enzyme in the liver needed to convert ammonia to urea. Ammonia then builds up to a toxic level, which can cause organ damage, particularly in the brain and the liver.
After years of preclinical research with similar disease-causing variants, Drs. Ahrens-Nicklas and Musunuru targeted KJ’s specific variant of CPS1, identified soon after his birth. Within six months, their team designed and manufactured a base editing therapy delivered via lipid nanoparticles to the liver in order to correct KJ’s faulty enzyme. In late February 2025, KJ received his first infusion of this experimental therapy, and since then, he has received follow-up doses in March and April 2025. In the newly-published New England Journal of Medicine paper, the researchers, along with their academic and industry collaborators, describe the customized CRISPR gene editing therapy that was rigorously yet speedily developed for administration to KJ.
As of April 2025, KJ had received three doses of the therapy with no serious side effects. In the short time since treatment, he has tolerated increased dietary protein and needed less nitrogen scavenger medication. He also has been able to recover from certain typical childhood illnesses like rhinovirus without ammonia building up in his body. A longer follow-up is needed to fully evaluate the benefits of the therapy.
“While KJ will need to be monitored carefully for the rest of his life, our initial findings are quite promising,” Dr. Ahrens-Nicklas said.
“We want each and every patient to have the potential to experience the same results we saw in this first patient, and we hope that other academic investigators will replicate this method for many rare diseases and give many patients a fair shot at living a healthy life,” Dr. Musunuru said. “The promise of gene therapy that we’ve heard about for decades is coming to fruition, and it’s going to utterly transform the way we approach medicine.”
A Future for KJ
Typically, patients with CPS1 deficiency, like KJ, are treated with a liver transplant. However, for patients to receive a liver transplant, they need to be medically stable and old enough to handle such a major procedure. During that time, episodes of increased ammonia can put patients at risk for ongoing, lifelong neurologic damage or even prove fatal. Because of these threats to lifelong health, the researchers knew that finding new ways to treat patients who are too young and small to receive liver transplants would be life-changing for families whose children faced this disorder.
“We would do anything for our kids, so with KJ, we wanted to figure out how we were going to support him and how we were going to get him to the point where he can do all the things a normal kid should be able to do,” his mother, Nicole Muldoon, said. “We thought it was our responsibility to help our child, so when the doctors came to us with their idea, we put our trust in them in the hopes that it could help not just KJ but other families in our position.”
“We’ve been in the thick of this since KJ was born, and our whole world’s been revolving around this little guy and his stay in the hospital,” his father, Kyle Muldoon, said. “We’re so excited to be able to finally be together at home so that KJ can be with his siblings, and we can finally take a deep breath.”
This study was supported by grants from the National Institutes of Health Somatic Cell Genome Editing Program (U01TR005355, U19NS132301), as well as additional National Institutes of Health grants (R35HL145203, U19NS132303, DP2CA281401, P01HL142494). In-kind contributions were received from Acuitas Therapeutics, Integrated DNA Technologies, Aldevron, and Danaher Corporation. Additional funding was provided by the CHOP Research Institute’s Gene Therapy for Inherited Metabolic Disorders Frontier Program.
Adapted from a Penn Medicine article by Matt Toal, May 15, 2025.
Medical Miracles at Penn Vet: State of the Art Care at Penn Vet’s New Bolton Center Saves Kale the Goat

One March morning, while at work, Allyson Bloodgood checked the remote camera on her farm. What she saw made her stomach drop. One of her one-month-old goat kids, Cabbage, had collapsed.
She rushed home, loaded Cabbage and his brother Kale, as a companion, into the car. They headed straight to Penn Vet’s New Bolton Center.
The brothers had a difficult start to life. Their mother died shortly after giving birth, and neither kid took well to the bottle. Ms. Bloodgood even bought another doe to adopt and feed them, but still, the little ones struggled. Nevertheless, she wasn’t prepared to see Cabbage so ill.
“We made it to New Bolton Center,” Ms. Bloodgood said. “Cabbage’s care team did everything they could, but sadly, he passed shortly after we arrived. Thankfully, they also examined Kale, even though he looked fine. Turns out, he was headed toward critical condition as well.”
The smaller, quieter Kale immediately became the focus of everyone’s attention.
Unseen Dangers
Kale appeared healthy. He drank from a bottle and was alert and active. But point-of-care bloodwork told a different story.
“Kale’s white blood cell count and blood sugar were low, both red flags in a neonate,” said Kavita Shroff, an internal medicine resident at Penn Vet. “With young animals, even the smallest abnormalities can signal something serious, and time is of the essence—things can go downhill quickly.”
The decreased white blood cells were concerning for a potential infection. Lungs, joints, and the gastrointestinal system and umbilicus are all places where infections can easily establish themselves in neonatal animals.
Kale was hospitalized and started on antibiotics. But, after a few days, his condition worsened even though follow-up blood work showed his white blood count had normalized.
“We conducted more diagnostics,” said Joy Tomlinson, an assistant professor of large animal medicine.
Digestive Detour
An ultrasound revealed the cause of Kale’s troubles: He was “rumen drinking.”
Goats have four stomach compartments—rumen, reticulum, omasum, and abomasum. In young goats, milk normally bypasses the rumen and goes straight to the abomasum, where it can be properly digested. This happens through a reflex that closes the esophageal groove during feeding. But when the groove fails to close—often due to illness or stress—milk enters the rumen instead, where it ferments and can cause serious complications.
“To stabilize Kale, we stopped all milk feedings and started total IV nutrition to deliver essential calories and nutrients directly into the bloodstream,” said Dr. Tomlinson. “We also began pain medication and stomach protectants to treat suspected abomasal ulcers, likely triggered by stress.”
But then another setback. Kale had a blood clot in his jugular vein. His care team quickly adjusted course, changing medications and starting anticoagulants.
Kale began to respond to treatment and slowly started to recover.
From Couch to Pasture
By early April, Kale was thriving. He went home more than twice the size he was on arrival.
“He lived with me inside the house at first,” Ms. Bloodgood said. “But then he started showing that goat spirit and had to get back with the herd.”
Today, Kale is outside with his friends, bounding through the pasture like a proper fainting goat. And Ms. Bloodgood is grateful to have access to specialized veterinary care.
Adapted from a Penn Vet article by Sacha Adorno, May 20, 2025.
Medical Miracles at Penn Vet: Penn Vet and Penn Medicine Collaborate on Canine Brain Surgery Using Cutting-Edge Augmented Reality Technology
 Geddy Lee has lived a big life for a little dog. As a puppy, the tiny terrier mix was abandoned in Mississippi during a high-speed car chase. Rescued by law enforcement, she found a loving home in Pennsylvania. Life was good—until last summer.
Geddy Lee has lived a big life for a little dog. As a puppy, the tiny terrier mix was abandoned in Mississippi during a high-speed car chase. Rescued by law enforcement, she found a loving home in Pennsylvania. Life was good—until last summer.
In August, Geddy Lee began having seizures, and her veterinarian referred the eight-year-old to Penn Vet for further evaluation. At Penn Vet’s Ryan Hospital, Tessa Arendt, a specialty intern in neurology, and Wojciech Panek, an assistant professor of neurology and neurosurgery in the department of clinical sciences and advanced medicine, performed a brain MRI, which revealed a right frontal lobe mass.
“An MRI doesn’t always tell us exactly what we’re dealing with, even though it allows us to see the tumor,” said Dr. Panek. “Based on Geddy Lee’s tumor imaging characteristics, we suspected a glioma—an aggressive brain tumor.”
Gliomas affect both dogs and humans. In people, the most malignant type, glioblastoma, carries a life expectancy of approximately 15-18 months with surgery, radiation, and chemotherapy. Without aggressive treatment, dogs typically survive for about a few months.
Geddy Lee’s owners, Michael and Erica Crotty, wanted to explore every option, including surgery. Surgery would also enable Dr. Panek to biopsy the tumor for a more exact diagnosis and to guide further therapeutic opportunities for Geddy Lee.
“It was amazing how much the team—all the doctors and nurses—rallied around her,” said Mr. Crotty. “It was so impressive that they pulled out all the stops—Erica and I said, do whatever you can for our girl.”
Geddy Lee’s procedure took place in September. She arrived at Ryan Hospital on a Monday. A week later, she would leave as the first dog to undergo successful canine brain surgery using cutting-edge augmented reality technology combined with infrared real-time guided resection.
Her case required a highly specialized team, including a veterinary neurosurgeon, radiologist, anesthesiologist, pathologist, nurses, and operating room technicians.
Joining the Penn Vet experts was a renowned neurosurgeon and brain tumor specialist from Penn’s Perelman School of Medicine: Presidential Associate Professor of Neurosurgery Nduka Amankulor, chief of neurosurgical oncology and director of the Penn Brain Tumor Center.
Drs. Amankulor and Panek partnered on Geddy Lee’s case, merging their world-class expertise in human and canine neurosurgery to push the boundaries of veterinary and human medicine.
“When I started at Penn, I approached Nduka because we face similar challenges,” said Dr. Panek, who has shadowed his colleague in human surgeries.
Added Dr. Amankulor, “One of the things that’s fascinating from a science perspective is that dogs develop brain tumors in a way that’s similar to humans, and genetically, the distribution of brain tumors in dogs is identical to humans.”
“This makes cases like Geddy Lee’s incredibly valuable for both veterinary and human science to advance the care mutually,” said Dr. Panek.
A Three-Pronged, Groundbreaking Approach
The primary goal of cancer excision—or debulking—surgery is to achieve “clean margins.” Even the tiniest remnant of cancerous tissue can increase the risk of local recurrence and disease spread, but gliomas infiltrate healthy brain tissue, making it extremely difficult to distinguish the tumor border.
Geddy Lee’s care team used a combination of advanced tools to ensure precise debulking.
“We combined several novel techniques to achieve the best possible tumor resection,” said Dr. Panek. “This was a unique three-part approach.”
A day before surgery, the pup received an injection of a special dye that glows under near-infrared light and accumulates in cancer cells. The imaging agent helps neurosurgeons better identify the limits of the glioma. Penn Vet’s David Holt, a professor of surgery, developed this technique to remove mammary and lung tumors from dogs.
The next day, Geddy Lee was placed under general anesthesia. The team performed a modified trans-frontal craniotomy, opening her skull for access to the brain. They then used three critical tools for guidance.
First, the surgical team broke new ground by employing an immersive augmented reality neuro-navigational system called VisAR to obtain optimal access to the tumor.
Developed by Novarad, the technology is used in human medical procedures for its precision and accuracy. It’s essentially, as Novarad describes it, “like a surgical GPS providing a road map to guide the surgeon through simple or complex surgeries.”
“The technology created a hologram from Geddy Lee’s initial MRI, which was really helpful for procedure planning and navigating hard-to-see areas,” said Dr. Panek. By superimposing Geddy Lee’s MRI images with anatomical accuracy onto the dog’s skull, the surgeons were able to first biopsy and then debulk the tumor.
“Penn Vet was the first in the world to use this technology in a canine surgery,” said Novarad CEO Wendell Gibby, the co-inventor of VisAR, who trained in neuroradiology at Penn Medicine. “Unlike traditional navigation, this system doesn’t just project an image on a 2D screen—it places the surgeon inside a fully immersive, 3D anatomical space co-registered to the patient. You can see areas inside the patient that you can’t see with the naked eye; hence augmented reality. The small footprint and lower cost mean that this technology can be widely used by veterinarians everywhere.”
Next for Geddy Lee was infrared imaging: “We shined an infrared camera on the brain, picking up the dye to map the tumor as we operated,” said Dr. Panek.
“Because infrared imaging is still relatively new, we also integrated intraoperative ultrasound,” he said. “This enabled us to see into the brain in real-time and confirm the infrared imaging was accurate and that we were removing the tumor to the best of our ability. The complex and delicate procedure took roughly five hours and was a success. Geddy Lee emerged from surgery unaware she had just made history.
“The way we approached her surgery highlights the value of state-of-the-art treatments that combine VisAR-guided biopsy and resection with infrared tumor imaging and complex genetic testing to guide the best next therapeutic steps,” Dr. Panek continued.
The Penn Vet lab of Timour Baslan would then perform a whole genome sequencing of Geddy Lee’s tumor.
“In veterinary medicine, the diagnostic process often ends after MRI imaging, and further treatment may be recommended based on those results alone. Many owners opt for radiation therapy or chemotherapy based on MRI findings, but without precise knowledge of the condition, these may not always be the best options.”
The following day, Geddy Lee was awake and charming her care team.
Soon after, she was discharged with medication to manage inflammation and prevent seizures. Follow-up visits showed that she was healing well. During a March re-evaluation at Ryan Hospital, Drs. Panek and Arendt were happy to report that Geddy Lee showed no radiological signs of tumor regrowth. Even better was the biopsy result from the Baslan lab.
“Geddy Lee’s genetic testing does not look like it is a glioma,” said Dr. Panek. “This is great news for the Crottys!” And for Geddy Lee, who will forever be a veterinary pioneer.
“In human medicine, we often have clearer answers because of extensive research and data,” said Dr. Panek. “But in veterinary medicine, we are still building our knowledge. Cases like Geddy Lee’s help validate our approach and provide insights for future studies in dogs and humans.”
For the Crottys, Geddy Lee’s contribution to science is a value-add: “She’s such a part of our family. There was never a question—we would do whatever it took to help her. And seeing her now, it was all worth it. She’s been through so much, and she just keeps going. She really is a survivor. We’ll always be grateful to the Ryan Hospital team. And we hope that research and technologies that come from Geddy Lee’s surgery can help, even if in a small way, to treat brain cancer.”
Another twist in Geddy Lee’s story: she is named after the lead singer of the band Rush, whose bandmate Neil Peart passed away in 2020 from brain cancer.
And for Dr. Amankulor, the entire experience was more than a professional highlight; it was personal. His father also died of the disease, inspiring him to move the needle on research. And, as a dog owner himself, he felt a personal tug during Geddy Lee’s surgery. “This was one of the most remarkable cases of my career,” he said. “I was emotional when Geddy Lee woke up, knowing what she means to her family. And her case is the beginning of something remarkable for science. It’s powerful, and we should all be proud of her contributions!”
Adapted from a Penn Vet article by Sacha Adorno, February 19, 2025.

 Thomas C. Childers, Jr., the Sheldon and Lucy Hackney Professor Emeritus in the department of history in the School of Arts & Sciences, died on November 7 after a long illness. He was 78.
Thomas C. Childers, Jr., the Sheldon and Lucy Hackney Professor Emeritus in the department of history in the School of Arts & Sciences, died on November 7 after a long illness. He was 78. Roger K. Raufer, Gr’84, an adjunct professor of city & regional planning in Penn’s School of Design (now the Weitzman School) and a lecturer in other departments around the University, including in computing, materials science & engineering, and legal studies & business ethics, died on August 2 after a battle with prostate cancer. He was 75.
Roger K. Raufer, Gr’84, an adjunct professor of city & regional planning in Penn’s School of Design (now the Weitzman School) and a lecturer in other departments around the University, including in computing, materials science & engineering, and legal studies & business ethics, died on August 2 after a battle with prostate cancer. He was 75.  The University of Pennsylvania’s Institute for Contemporary Art (ICA) and WXPN radio station have received Pew creative project grants. Additionally, filmmaker Sosena Solomon, who teaches in the department of cinema and media studies in the School of Arts & Sciences, has been awarded a 2025 Pew Fellowship in the Arts.
The University of Pennsylvania’s Institute for Contemporary Art (ICA) and WXPN radio station have received Pew creative project grants. Additionally, filmmaker Sosena Solomon, who teaches in the department of cinema and media studies in the School of Arts & Sciences, has been awarded a 2025 Pew Fellowship in the Arts. Deep Jariwala, an associate professor and the Peter and Susanne Armstrong Distinguished Scholar in Electrical and Systems Engineering in Penn Engineering, has been elected to the 2026 class of Optica Fellows, a distinction granted to no more than 10 percent of the society’s membership and reserved for researchers who have made outstanding contributions to the fields of optics and photonics.
Deep Jariwala, an associate professor and the Peter and Susanne Armstrong Distinguished Scholar in Electrical and Systems Engineering in Penn Engineering, has been elected to the 2026 class of Optica Fellows, a distinction granted to no more than 10 percent of the society’s membership and reserved for researchers who have made outstanding contributions to the fields of optics and photonics.  Antonio Loquercio, an assistant professor in electrical systems and engineering and in computer and information science in Penn Engineering, was awarded the 2025 ISSNAF Young Investigator Mario Gerla Award for his research in “enhancing the performance of complex robotic systems by focusing on the pivotal role of perception in building effective world models for decision-making.” The award, established in memory of the late Mario Gerla, a pioneer of computer networking and a founding member of ISSNAF, acknowledges early-career researchers who are making transformative advances in computer science.
Antonio Loquercio, an assistant professor in electrical systems and engineering and in computer and information science in Penn Engineering, was awarded the 2025 ISSNAF Young Investigator Mario Gerla Award for his research in “enhancing the performance of complex robotic systems by focusing on the pivotal role of perception in building effective world models for decision-making.” The award, established in memory of the late Mario Gerla, a pioneer of computer networking and a founding member of ISSNAF, acknowledges early-career researchers who are making transformative advances in computer science. Flavia Teles, a professor in the department of basic & translational sciences in Penn Dental Medicine, has been honored for her periodontal research as the recipient of the American Academy of Periodontology (AAP)’s 2025 Clinical Research Award.
Flavia Teles, a professor in the department of basic & translational sciences in Penn Dental Medicine, has been honored for her periodontal research as the recipient of the American Academy of Periodontology (AAP)’s 2025 Clinical Research Award.
 University of Pennsylvania graduate student Adelaide Lyall and senior Norah Rami have been chosen as 2026 Marshall Scholars. Established by the British government, the Marshall Scholarship funds as much as three years of study for a graduate degree in any field in an institution in the United Kingdom.
University of Pennsylvania graduate student Adelaide Lyall and senior Norah Rami have been chosen as 2026 Marshall Scholars. Established by the British government, the Marshall Scholarship funds as much as three years of study for a graduate degree in any field in an institution in the United Kingdom.




 Geddy Lee has lived a big life for a little dog. As a puppy, the tiny terrier mix was abandoned in Mississippi during a high-speed car chase. Rescued by law enforcement, she found a loving home in Pennsylvania. Life was good—until last summer.
Geddy Lee has lived a big life for a little dog. As a puppy, the tiny terrier mix was abandoned in Mississippi during a high-speed car chase. Rescued by law enforcement, she found a loving home in Pennsylvania. Life was good—until last summer.