Medical 'Miracles on 34th Street'
Brain Remaps Itself in Child with Double Hand Transplant
The first child to undergo a successful hand transplant is also the first child in whom scientists have detected massive changes in how sensations from the hands are represented in the brain. The brain reorganization is thought to have begun six years before the transplant, when the child had both hands amputated because of a severe infection during infancy. After he received transplanted hands, his brain reverted toward a more typical pattern.
Each area of the body that receives nerve sensations sends signals to a corresponding site in the brain. The spatial pattern in which those signals activate the brain’s neurons is called somatosensory representation—particular parts of the brain reflect specific parts of the body.
“We know from research in nonhuman primates and from brain imaging studies in adult patients that, following amputation, the brain remaps itself when it no longer receives input from the hands,” said first author William Gaetz, a radiology researcher in the Biomagnetic Imaging Laboratory at Children’s Hospital of Philadelphia (CHOP). “The brain area representing sensations from the lips shifts as much as two centimeters to the area formerly representing the hands.”
This brain remapping that occurs after upper limb amputation is called massive cortical reorganization (MCR). “We had hoped to see MCR in our patient, and indeed, we were the first to observe MCR in a child,” said Dr. Gaetz. “We were even more excited to observe what happened next—when the patient’s new hands started to recover function. For our patient, we found that the process is reversible.”
Researchers from CHOP and the Perelman School of Medicine at the University of Pennsylvania published their findings in the Annals of Clinical and Translational Neurology. Their case report described Zion Harvey, now 10 years old, who received worldwide media coverage two years ago as the first child to undergo a successful hand transplant (Almanac December 15, 2015).
A 40-member team led by L. Scott Levin, chairman of orthopaedic surgery and a professor of plastic surgery at Penn Medicine, and director of the Hand Transplantation Program at CHOP, performed that milestone surgery in July, 2015 at CHOP. “Zion has been a child of many firsts here at Penn Medicine and Children’s Hospital of Philadelphia, and across the world,” said Dr. Levin, senior author of the paper. He added, “With the changes observed in his brain, which our collaborative team has been closely evaluating since his transplant two years ago, Zion is now the first child to exhibit brain mapping reorientation. This is a tremendous milestone not only for our team and our research, but for Zion himself. It is yet another marker of his amazing progress, and continued advancement with his new limbs.”
The researchers used magnetoencephalography (MEG), which measures magnetic activity in the brain, to detect the location, signal strength and timing of the patient’s responses to sensory stimuli applied lightly to his lips and fingers. They performed MEG four times in the year following the bilateral hand transplant, performing similar tests on five healthy children who served as age-matched controls.
At the first two visits, Zion’s fingertips did not respond to tactile stimulation—being touched with a thin filament. When experimenters touched the patient’s lips, the MEG signal registered in the hand area of the brain’s cortex, but with a delay of 20 milliseconds compared to controls. At the two later visits, MEG signals from lip stimulation had returned to the lip region of the brain, with a normal response time—an indication that brain remapping was reverting to a more normal pattern.
When experimenters touched Zion’s fingertips in the two later visits, the MEG signals appeared in the hand region of the brain, with a shorter delay in response time from visit 3 to visit 4, but with higher-than-normal signal strength. “The sensory signals are arriving in the correct location in the brain, but may not yet be getting fully integrated into the somatosensory network,” said Dr. Gaetz. “We expect that over time, these sensory responses will become more age-typical.”
Dr. Gaetz added, “These results have raised many new questions and generated excitement about brain plasticity, particularly in children. Some of those new questions include, what is the best age to get a hand transplant? Does MCR always occur after amputation? How does brain mapping look in people born without hands? Would we see MCR reverse in an adult, as we did in this patient? We are planning new research to investigate some of these questions.”
Zion’s progress provides encouraging details on his functional abilities. “Our follow-up studies 18 months after this transplant showed that he is able to write, dress and feed himself more independently than before his operation—important considerations in improving his quality of life,” said Dr. Levin.
A Transplant and a Cure: Penn Team Eradicates Hepatitis C in 10 Patients Following Lifesaving Transplants from Infected Donors
Ten patients at Penn Medicine have been cured of the Hepatitis C virus (HCV) following lifesaving kidney transplants from deceased donors who were infected with the disease. The findings point to new strategies for increasing the supply of organs for the nation’s more than 97,000 patients who are awaiting kidney transplants—often for as many as five or more years.
In 2016, Penn Medicine launched an innovative clinical trial to test the effect of transplanting kidneys from donors with HCV into patients currently on the kidney transplant waitlist who do not have the virus, and who opt in to receive these otherwise unused organs. Recipients were then treated with an antiviral therapy in an effort to cure the virus. Early data from the study were presented by David S. Goldberg, an assistant professor of medicine and epidemiology in the Perelman School of Medicine at Penn, at the 2017 American Transplant Congress in Chicago, and were simultaneously published in the New England Journal of Medicine.
“We started this trial in the hopes that, if successful, we could open up an entirely new pool of donor organs, and effectively transplant hundreds, if not thousands, more patients who are awaiting a lifesaving organ,” Dr. Goldberg said. “Historically, Hepatitis C-infected kidneys were often discarded, and were thought to be damaged or too ‘high-risk.’ Our pilot data demonstrate the ability to cure the contracted virus following transplantation in this patient population. If future studies are successful, this may be a viable option for patients who may otherwise never see a transplant.”
Dr. Goldberg, who co-led the study with Peter Reese, an assistant professor of medicine and epidemiology at Penn Medicine and chair of the Ethics Committee for the United Network of Organ Sharing (UNOS), approached and enrolled participants who relied on dialysis treatments to stand in for their damaged kidneys. Participants were between 40 and 65 years of age and had been waiting for a transplant for at least a year and a half. A three-step process of education and consent was used during pre-enrollment to ensure patients, and their loved ones were provided with a comprehensive understanding of the risks. Once enrolled, and as organs became available, the team performed HCV donor genotyping during the allocation process, selecting only kidneys that were considered “high quality.”
In the first phase of the study, to date, 10 patients have received transplants using the protocol. On average, patients received a transplant 58 days after enrolling in the trial—some in as quickly as 11 days, while others waited for more than 100 days. At three days after surgery, patients were tested for HCV, and all 10 tested positive for the disease. Next, the participants were treated with the standard 12-week course of elbasvir/grazoprevir, commonly known as Zepatier, a recently-approved and highly effective oral medication prescribed to eradicate HCV. All 10 patients have been cured of their contracted HCV.
“For so long, HCV was a virus with a very negative stigma associated with it, especially among physicians. So it was interesting to see that patients were quick to jump at the chance to get this transplant, despite the possibility that they could get Hepatitis C permanently,” Dr. Reese said. “Going into the study, we knew it was a possibility that some or all of the patients would contract HCV, and that they could have the disease for the rest of their lives if we were unsuccessful. But for these patients, getting off of dialysis and getting back to their normal lives was very much worth the risk.”
Following the early positive results, the research team was granted an extension of their study, which will allow them to transplant and treat an additional 10 patients—20 patients in total.
The research team is designing a new clinical trial that will study this same approach in patients who are heart-transplant recipients, and in the future they hope to examine the efficacy of this approach in liver and lung transplants. Researchers note there is a need for longer and larger trials to continue evaluating the effectiveness of HCV-positive to HCV-negative transplantation followed by antiviral therapy in a broader population.
Additional Penn Medicine experts involved in this study span disciplines including infectious diseases, transplantation surgery, gastroenterology, hepatology, and pathology and laboratory medicine, including Deirdre Sawinski, Roy Bloom, Raj Reddy, Emily Blumberg, Jennifer Trofe-Clark, Vivianna Van Deerlin, Midhat Farooqi, Peter Abt, Matthew Levine, Paige Porrett, Susanna Nazarian, Ali Naji, Maureen McCauley and Anna Sicilia. The study is supported by a research grant from the Merck Investigator Initiated Studies Program, and Merck supplied the antiviral drugs used in the study.
and, at Penn Veterinary Medicine
Note: These stories are abbreviated versions of articles from Penn Vet Extra. View the full stories. —Eds.
Comprehensive Cancer Care at Ryan Hospital

Dingus is undergoing Comprehensive Cancer Care, a cross-disciplinary approach to the diagnosis and treatment of cancer in pets
Dingus, a 17-year-old cat, was already being treated at Ryan Hospital for small cell gastrointestinal (GI) lymphoma. Diagnosed in November 2016, he had responded well to medication, but through the following summer he was slowly losing weight. He came back to Ryan for an examination where an abdominal ultrasound showed his intestinal tract was normal, but revealed something else. He had a mass in one of his lung lobes.
Dingus is a remarkable cat with an extensive medical history and two very loving owners, Christopher Lengner and Heather Steinman. Not only is Dr. Lengner Dingus’ owner, he is an associate professor of biomedical sciences at Penn Vet, and a cancer researcher. “On one hand,” said Dr. Lengner, “I’m the owner of a cat with cancer; on the other, I’m a cancer researcher.”
Dingus’ other owner, Dr. Steinman, is a former cancer researcher, and is now a vice president at the Wistar Institute where she continues to focus on bringing new cancer therapies, including tumor vaccines and immunotherapies, to patients.
In 2011, Ryan clinicians removed Dingus’ left eye due to uveitus and glaucoma caused by an infection. Then in 2014, he developed a benign tumor in his right ear, which necessitated the removal of the ear canal. In addition to lymphoma, he also has heart disease and chronic lower airway disease.
Dingus’s owners discussed surgery for Dingus and agreed to proceed, as his quality of life was still very good.
Beth Callan, professor of internal medicine and Dingus’ primary vet, spoke with James Perry, assistant professor of surgical oncology, the newest member of the Comprehensive Cancer Care team, and he agreed to take Dingus’ case. Comprehensive Cancer Care is a cross-disciplinary approach to the diagnosis and treatment of cancer in pets. Ryan clinicians provide a comprehensive assessment of each patient’s cancer care needs within one appointment. They work with clients through the diagnosis and subsequent treatment plan for their pets, be it chemotherapy, surgery, radiation therapy, or supportive care.
There is the notion that cancer can be treated by differentiating the cells and not killing them. If the cancer is a stem cell that divides uncontrollably and the cell can be driven to differentiate, it will exit the cell cycle and stop dividing, alleviating the disease.
“In normal tissue, stem cells are resistant to injury and repopulate the tissue in the face of injury, which is a good thing,” said Dr. Lengner. “When you irradiate a colorectal tumor, by all measures it’s gone, but five or ten years later it comes back, and it comes back in the exact same place. Those cells need to be identified, purified, and studied, so that’s really what we’re interested in.”
Dr. Perry led the surgery, assisted by Dr. Ludwig and Intern Julie Pfeifer. Instead of cutting through the sternum, Perry was able to remove the affected lung lobe through the muscle between Dingus’ ribs. This approach reduced his pain, healing time, and hospital stay.
The Penn Vet Cancer Center is a new initiative that will bring together basic cancer research, clinical trials, and patient care into one centralized location at Ryan Hospital. In this state-of-the-art facility, scientists and clinicians will be able to bring research breakthroughs directly to patients. Dr. Lengner hopes there will be a push to bring molecular diagnostics into a clinical setting with the intent of identifying genetic mutations within companion animals, particularly as it relates to cancer.
“If we knew the genetic basis of a lot of these cancers that occur in companion animals, we could easily start testing these next generation drugs under development for human cancer, including many coming out of Penn Medicine’s Abramson Cancer Center,” he said. “Hopefully Penn Vet’s new Cancer Center will embrace all the knowledge from the human side and try to translate it into companion animals. This is a personal thing for me.”
Prosthetic for Pete the Parrot
Benjamin Spalding was working late when he heard the screams.
He ran outside to investigate and saw that a fox had startled Pete, his 34-year-old Mealy Amazon parrot. As Pete climbed up the side of the backyard aviary, the fox grabbed his foot and tore it off.
Mr. Spalding and his wife, Stacey Gehringer, immediately put Pete into his carry cage, got in the car, and headed to an emergency veterinary clinic nearby. Ms. Gehringer called the clinic to let them know they were on the way, but the clinic said they couldn’t take Pete as a patient. There are few veterinary hospitals close to the Lehigh Valley with experienced Exotics vets on staff. Fortunately, Penn Vet’s Ryan Hospital is one of them. When Ms. Gehringer called Ryan’s Emergency Service, she was told to bring Pete in.
La’Toya Latney, service head and attending clinician of the Exotic Companion Animal Medicine service, was on call when she received the 2 a.m. phone call. When she arrived at the hospital, Pete seemed alert, despite having lost a lot of blood. Dr. Latney’s primary goal was to stop the bleeding and provide fluid therapy.
Despite the blood loss, Pete’s odds of survival were promising, as birds have a unique ability to reproduce red blood cells much faster than humans. It’s been shown that birds can lose 30% of their total blood volume without showing signs of shock. Birds would have to sustain about a 60% loss of blood before there would be a notable change in blood pressure or signs of decompensation.
“Dr. Latney’s demeanor with her patients is amazing,” said Mr. Spalding. “I’ve been to several different vets and they all treated Pete with fear. He’s never seen anything short of a kiss at Ryan Exotics!”
Having addressed the relatively short-term goal of closing the wound on Pete’s stump, there were some long-term complications to take into consideration.
“Given that Pete is a larger-bodied bird, he could experience long-term pain if we don’t provide some type of comparative support,” said Dr. Latney.
Never one to back away from a challenge, Dr. Latney reached out to Jonathan Wood, staff veterinarian in neurology and neurosurgery, with the task of designing a prosthetic leg for Pete.
Dr. Wood met with Stephen Smeltzer, digital fabrication manager at PennDesign’s Fabrication Lab, to examine the CT scan and formulate a plan. Mr. Smeltzer asked questions about birds and bird bones, the weight and stiffness of the prosthetic, and how they might attach it. He then drew sketches.
“One of the things we love about working with Penn Vet is seeing our technology have an immediate impact in the world,” said Mr. Smeltzer.
The team tried two different prototypes for Pete, but neither were secure enough to support his weight. A third design is currently being printed at the Fabrication Lab and should be ready for Pete to try out soon.
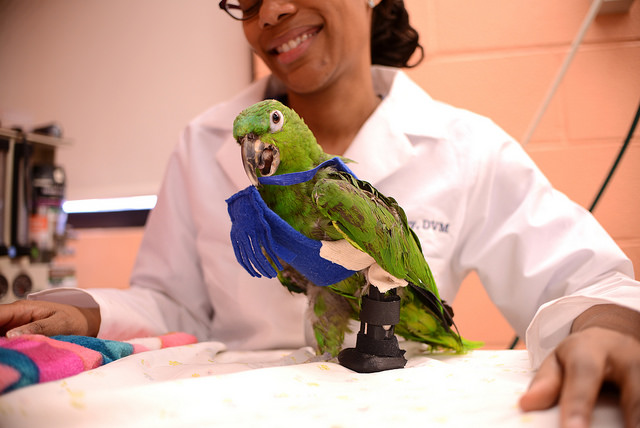
La'Toya Latney is working with Pete to find the perfect prosthetic leg.
Drs. Wood and Latney are currently working on an attachment system that is safe and comfortable for Pete. They estimate two to three months before the final fitted model is ready.
“In the meantime, we’ve encouraged Pete’s owners to do physical therapy on the remaining limb,” said Dr. Latney.
Even one-legged, Pete is enjoying a full range of activity at home, including climbing. Everyone involved is eager to finalize the prosthetic attachment system to give Pete an even better quality of life at home.



















 Download the AT PENN Calendar
Download the AT PENN Calendar

